The effect of ionophone use and essential oil compounds in calf diets on animal growth and the prevalence of antibiotic resistant Escherichia coli
Estimated reading time: 10 minutes
- The aim of the study was to determine if either monensin or a blend of essential oil compounds could yield similar health and growth benefits while influencing the prevalence of antibiotic resistant Escherichia coli (E.coli).
- Due to the increased concerns over antimicrobial resistant (AMR) pathogens, the use of ionophores in animal nutrition has received scrutiny and led to the ban of all growth promoting antimicrobial drugs in the European Union in 2006.
- Essential oils (EOs) are complex phenolic and volatile compounds produced by herbal plants as secondary metabolites. These EO compounds have been studied for their anticoccidial and antibacterial properties.
- Overall growth and health benefits associated with either a diet containing monensin or a blend of capsaicin, carvacrol, cinnamaldehyde and garlic extract were similar. No benefits were associated with either diet as no decrease in Eimeria spp. oocysts, and no increased rumen development could be linked to any of the diets.
The need for alternative growth promoting and disease preventing feed additives to ionophores in calf starter feed, has become an area of interest in many European Union (EU) countries since the ban of the subtherapeutic use of antimicrobial drugs.
The World Health Organisation considers antimicrobial resistance as one of the three largest threats to human health. Increased legislation on antibiotic use in the livestock sectors of many countries and concern of a rise in antibiotic resistant pathogens urged the investigation into alternatives to ionophores such as essential oil compounds.
The aim of the study was to determine if either monensin or a blend of essential oil compounds could yield similar health and growth benefits while influencing the prevalence of antibiotic resistant Escherichia coli.
Background
Ionophores are antimicrobial compounds used in calf nutrition to control coccidiosis and aid in growth and health of the calf (Baldwin et al., 2003; Tahmasbi et al., 2014). Early nutrition and management practices are crucial for growth, health and productivity of both replacement heifers and surplus veal calves (Tahmasbi et al., 2014), and monensin has been readily acknowledged for its anticoccidial and growth promoting effects (Edrington et al., 2003; Van Baale et al., 2004).
Due to the increased concerns over antimicrobial resistant (AMR) pathogens, the use of ionophores in animal nutrition has received scrutiny and led to the ban of all growth promoting antimicrobial drugs in the European Union in 2006 (Sánchez et al., 2021). Furthermore, European pharmaceutical companies are phasing out growth promoting antimicrobial drugs, which sparked conversation among researchers to investigate alternative non-antibiotic feed additives (National Department of Health, 2021).
Essential oils (EOs) are complex phenolic and volatile compounds produced by herbal plants as secondary metabolites (Spisni et al., 2020). These EO compounds have been studied for their anticoccidial and antibacterial properties. Carvacrol, capsaicin, cinnamaldehyde and thymol have shown promising growth-modulating action in lambs, poultry and piglets (Castillo et al., 2012; Spisni et al., 2020).
Furthermore, EO compounds have often been overlooked in calf nutrition and as a result limited research is available on the practical application of an EO compound blend that, when added to the diet, could successfully be compared to ionophores to aid in health and growth performance of pre-weaned calves (Salazar et al., 2019).
Calves that received a garlic or oregano EO supplement were observed to have decreased morbidity and increased milk intake (Ghosh et al., 2010; Tapki et al., 2020; Chen et al., 2021). Calves that received a thymol and eugenol EO showed increased weight gain and nutrient absorption in the gastrointestinal tract (GIT) (Kazemi-Bonchenari et al., 2018; Kekana et al., 2020).
Antibiotic resistance can be defined as the ability of a bacteria to withstand an antibiotic agent designed to kill or inhibit the growth of the bacterium by targeting specific processes that are essential for its survival (Simjee, 2019). Livestock farming practices are often seen as reservoirs for antibiotic resistant (ABR) pathogenic bacteria that can impact both human and animal health.
High levels of therapeutic or subtherapeutic antibiotic use is a common practice in dairy operations and exposure to the antibiotics have been shown to alter the microbiota in the GIT of neonatal calves (Pardon et al., 2012; Manishimwe et al., 2017). Furthermore, calf rearing systems have been identified as one of the sectors with the highest abundance of ABR bacteria (Amin and Seifert, 2021).
Although ionophores are not considered as medically important antimicrobial drugs, animal-only drugs can lead to the resistance of pathogenic bacteria to medically important antibiotics through shared resistance mechanisms, or resistant gene transfer (Alarcon and Omenaca, 2004; Wong, 2019).
Read more about alternative soya byproducts in animal feed.
Materials and methods
Treatments and animal management
The trial was conducted on two farms in the Western Cape of South Africa. Jersey bull calves (n = 24; Farm A) and Ayrshire heifer calves (n = 39; Farm B) were randomly allocated to one of three treatment groups: (CON) no added elements to either the liquid or solid diet; (EOC) added garlic extract (0,6g/day per calf) to the liquid diet and an added blend of cinnamaldehyde, carvacrol and capsaicin (150mg/kg dry matter; DM) to the starter feed diet; and (MON) no added elements to the liquid diet, and added monensin (30mg/kg DM) in the starter
feed diet.
All calves received veterinary treatment if necessary, and all antibiotic administration and supportive therapy (electrolytes, anti-inflammatory medication, etc.) as well as disease status were fully recorded throughout the study. Intake and faecal scores were recorded daily, whereas bodyweight of the individual calves was taken weekly, and rectal swabs were taken on day 1, 30 and 56 of the trial.
Furthermore, faecal samples of individual calves were obtained daily between 20 to 40 days of age to determine the prevalence of Eimeria spp. oocysts between the treatment groups by using a modified McMaster technique (LNE10-300; Zajac et al., 2010). Furthermore, eighteen Jersey bull calves were slaughtered at 60 days of age to determine rumen wall thickness, papillae density, rumen papillae length (PL) and rumen papillae width (PW).
Faecal swabs were taken for the isolation of E. coli whereafter a Kirby-Bauer disc diffusion method was used to determine antibiotic resistant isolates (Humphries et al., 2018; Van den Honert et al., 2021). Isolates were tested for resistance to sulfamethoxazole-trimethoprim, chloramphenicol, ampicillin, tetracycline, ceftazidime and streptomycin.
The zone diameters around each antibiotic (Figure 1) were measured to determine susceptibility of the isolate to the respective antibiotic based on the Clinical and Laboratory Standards Institute (2022) zone diameter interpretive standards.
Discussion
Animal growth, health and rumen development
The addition of a pungent garlic extract in the milk or MR did not affect the milk intake of the calves. Similar starter feed intake, average daily gain, and feed conversion ratio between the three diets were observed for both the Jersey bull calves and the Ayrshire heifer calves.
Eimeria spp. oocysts were present in 66,7% of Jersey bull calves and all the oocyst counts were within normal ranges, 16 to 1447 oocysts per gram of faeces (OPG; Makau et al., 2017; Lopez-Osorio et al., 2020). The addition of either an EO compound blend or monensin to the diet of the bull calves had no significant effect on the number of oocysts present (P = 0,214).
Rumen papillae density, PL and PW were less than indicated in literature
for calves aged 60 days (Ragionieri
et al., 2016). It can be theorised that high frequency of disease may have had an effect on overall nutrient absorption and therefore subsequent growth and rumen development (Baldwin et al., 2003). Furthermore, the diet had no effect on rumen papillae density, PL or PW.
Antibiotic resistant E. coli
E. coli is abundant in soil, water and the GIT of all livestock (Amin and Seifert, 2021; Formenti et al., 2021; Martínez-Vázquez et al., 2021) and can transfer antibiotic resistance elements (genes) to other gram-negative bacteria (Alarcon and Omenaca, 2004). It was therefore regarded as a representative indicator of antibiotic resistance of gram-negative bacteria in this study (Gregova and Kmet, 2020).
There was no diet influence on the prevalence of E. coli in the faecal matter of the calves (P = 0,09). Furthermore, 32% of the isolates were resistant to at least one or more of the antibiotics tested in the study, 10% indicated intermediate resistance, and 58% were susceptible to the antibiotics tested (sulfamethoxazole-trimethoprim, chloramphenicol, ampicillin, tetracycline, ceftazidime and streptomycin).
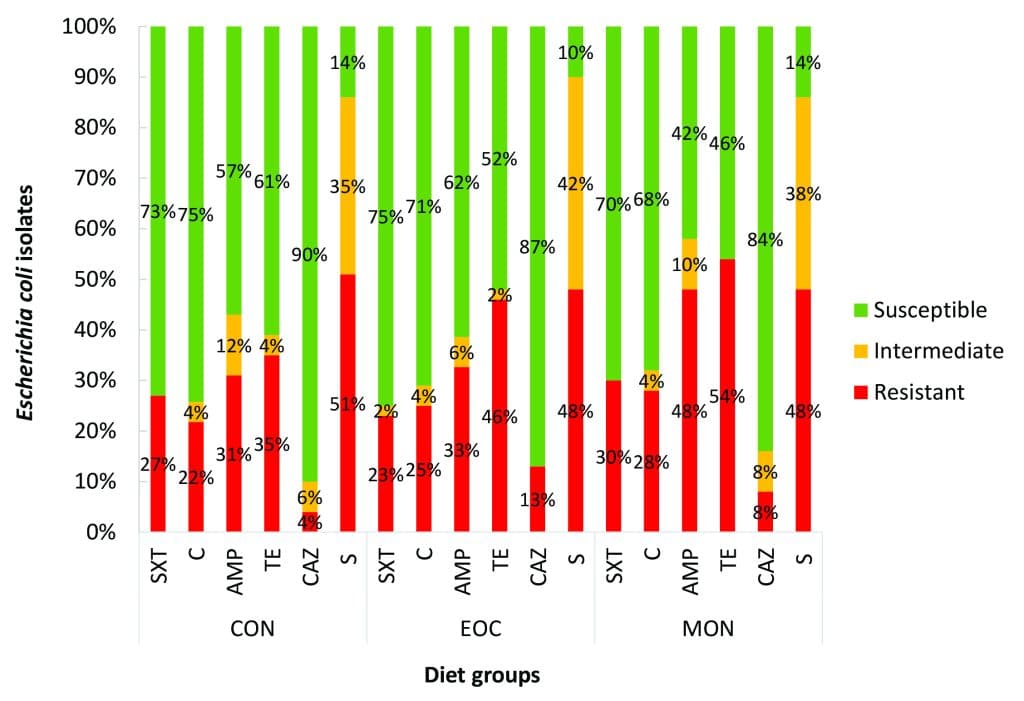
Figure 2 provides a complete summary of the antibiotic resistance profiles across all the isolates that were tested against antibiotics commonly used in the livestock sector. Isolates from the CON diet group displayed the most resistance to streptomycin (51%) followed by tetracycline (35%) and ampicillin (31%). The EOC diet resulted in a similar resistance pattern, but a slightly increased resistance to tetracycline (54%) and ampicillin (48%) were observed from the MON diet group.
Multidrug resistant E. coli
Antibiotic resistance classification considers the level of resistance of ABR isolates, where multidrug resistant (MDR) isolates are resistant to three or more different classes of antibiotics. To further understand the impact the diets may have on antibiotic resistance, only the resistant E. coli isolates were further observed for multidrug resistance.
An increase in MDR E. coli (P = 0,02) was observed for the MON diet compared to the EOC and CON diets (Figure 3). These findings suggest that even though no increase in the prevalence of general ABR E. coli were found, isolates from the MON fed group did indicate resistance to more classes of antibiotics than the EOC and CON diet groups.
This contradicts previous research with the theory that subtherapeutic use of antibiotics have no effect on antibiotic resistance (Thames et al., 2012), but rather supports the current hypothesis that ionophores included in calf starter diets result in increased antibiotic resistance of E. coli.
Conclusion
Overall growth and health benefits associated with either a diet containing monensin or a blend of capsaicin, carvacrol, cinnamaldehyde and garlic extract were similar. No benefits were associated with either diet as no decrease in Eimeria spp. oocysts, and no increased rumen development could be linked to any of the diets. However, increased multidrug resistant E. coli was associated with a diet that contains monensin, which further confirms the theory that ionophores do contribute to the increased burden of antibiotic resistance at farm level.
Figure 1: Mueller-Hinton agar plates with antibiotic discs (A) after a 24 hour incubation period; B and C display the zones around the antibiotic discs, the diameter of the zone would be measured to determine resistance (i.e., zone C would be measured as 0mm, and classified resistant).
Figure 2: Representation of the Kirby-Bauer disc diffusion susceptibility test results of all isolates from South African dairy calves (Jersey and Ayrshire) fed one of three diets.
Figure 3: Averaged antibiotic resistance classification of the dairy calves across three diet groups for E. coli (P ≤ 0,05) where R = resistant and MDR = multidrug resistant.
Michelle Gouws formulated and executed this article as part of her MScAgric research thesis. Dr L Steyn was responsible for the supervision and development of the research. Gouws graduated with her MScAgric Animal Sciences degree in March 2023, and is currently pursuing a PhD in Agriculture at Stellenbosch University. – Michelle Gouws and Louwrinda Steyn, Stellenbosch University
References available on request. For more information, send an email to Michelle Gouws at 19903812@sun.ac.za.